

Rakhoi TV là kênh xem bóng đá quen thuộc hàng đầu với những người yêu thích thể thao vua. Trên RakhoiTV bạn sẽ tìm thấy được đam mê của mình, hòa mình vào những trận cầu nảy lửa, sự sôi động trên khán đài. Những khoảnh khắc vỡ òa trên sân cỏ sẽ được truyền tải một cách chân thực nhất, tạo nên những giây phút đáng nhớ.
Mục lục

RakhoiTV, một trang web hàng đầu về phát sóng trực tiếp bóng đá tại Việt Nam, ra đời để đáp ứng sự đam mê ngày càng lớn của người hâm mộ bóng đá trong nước. Với nền tảng này, người hâm mộ không còn phải bất tiện khi theo dõi trận đấu, mà thay vào đó, có thể dễ dàng theo dõi mọi trận đấu từ khắp nơi trên thế giới thông qua các đường link trực tuyến được cung cấp.
Trước đây, việc theo dõi bóng đá đòi hỏi người hâm mộ phải tìm kiếm trên các trang web trả phí hoặc xem trên truyền hình, việc này gây mất thời gian và bất tiện. Rakhoi TV thấu hiểu điều đó nên đã cung cấp những đường link đáng tin cậy, an toàn, giúp người xem bóng đá trực tuyến dễ dàng chọn lựa trận đấu hoặc giải đấu theo lịch thi đấu được cung cấp trên trang web.
Bên cạnh theo dõi giải đấu, Rakhoitv còn là nơi người hâm mộ có thể hỗ trợ anh em tham gia cá cược và có cơ hội nhận được những phần thưởng hấp dẫn. Trang chủ của kênh cung cấp thông tin nhận định, soi tỷ lệ kèo bóng đá của chuyên gia lâu năm, giúp người xem đưa ra dự đoán chuẩn, cơ hội lớn để chiến thắng.

Đến với địa chỉ thể thao hàng đầu này, bạn sẽ được tận hưởng những giải đấu hấp dẫn hàng đầu, điển hình như:
Với những tính năng hấp dẫn, cuốn hút anh em sẽ được tận hưởng những điều thú vị tại đây như:

Hiện nay, RakhoiTV hiện đang cung cấp đầy đủ những bảng xếp hạng của đầy đủ mọi giải đấu diễn ra trên khắp thế giới. Người xem có thể theo dõi số điểm đạt được, hiệu số thắng/thua, số vòng thi đấu đã diễn ra và còn lại, đánh giá khả năng ghi bàn và không bị ghi bàn của từng câu lạc bộ.
Thành tích gần đây trong 5 trận đấu cũng là một chỉ số quan trọng giúp đánh giá form của đội bóng. Những thông tin trên hệ thống được cập nhật liên tục và được kiểm duyệt, đảm bảo tính tin cậy cao.
Kết quả trận đấu trên Ra Khoi TV là nguồn thông tin chi tiết về tỷ số và kết quả của mọi trận cầu đỉnh cao trên thế giới. Người hâm mộ dễ dàng, nhanh chóng cập nhật kết quả trực tuyến, biết đội nào đã thắng, thua hoặc hòa. Điều này giúp bạn luôn kịp thời theo dõi tình hình phát triển của các trận đấu yêu thích và trải nghiệm một cách tiện lợi, đáng tin cậy.
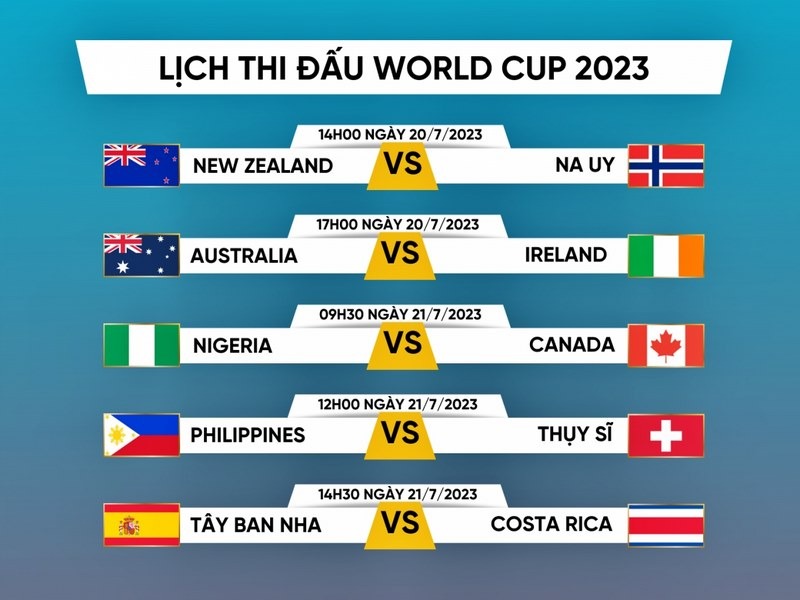
Web bóng đá còn chia sẻ những thông tin về Lịch Thi Đấu. Đây là một phần quan trọng giúp người hâm mộ biết trước thời gian, ngày và sân vận động khi những cuộc so tài diễn ra. Điều này hỗ trợ người dùng có thể chủ động để theo dõi trận đấu, bố trí thời gian để đắm mình trong không gian thể thao.
Thông tin kèo nhà cái là đặc biệt dành cho những người yêu thích cá cược bóng đá. rakhoitv cung cấp thông tin chi tiết về kèo nhà cái, bài phân tích và đánh giá từ các chuyên gia nhiều kinh nghiệm. Điều này giúp anh em bet thủ có được những cái nhìn tổng quan, đưa ra những lựa chọn thông minh khi xuống tiền.
Đây là một tính năng độc đáo giúp người dùng xem lại những đường bóng nổi bật ngay sau khi trận đấu kết thúc Highlight bóng đá đặc biệt hữu ích cho những người có lịch trình bận rộn, không thể theo dõi trọn vẹn trận đấu, nhưng vẫn muốn không bỏ lỡ những tình huống quan trọng trong trận đấu.
Rakhoi TV tận dụng công nghệ để mang đến trải nghiệm xem bóng đá linh hoạt và tiện ích cho cộng đồng người hâm mộ. Bạn sẽ không bỏ lỡ bất cứ thông tin quan trọng nào, mọi khoảnh khắc đều được tổng hợp một cách trọn vẹn nhất.
Để xem những trận cầu đỉnh cao, bạn hãy nhanh chóng thực hiện theo những thao tác đơn giản sau đây:

Dưới đây là những vấn đề mà bạn cần lưu ý khi xem bóng đá tại kênh để có được một trải nghiệm mượt mà nhất:
Lưu ý luôn chọn các đường link Rakhoi TV chính chủ từ trang web để đảm bảo bạn đang kết nối với nguồn đáng tin cậy, tránh rủi ro từ các trang web không chính thức. Đường link chính chủ thường mang lại độ an toàn cao và chất lượng hình ảnh, âm thanh tốt nhất.
Trước khi xem trận đấu tại kênh Rakhoi TV, hãy kiểm tra kết nối mạng của thiết bị để đảm bảo đường truyền trong trạng thái ổn định. Kết nối yếu có thể gây giật lag hoặc mất kết nối, làm giảm chất lượng trải nghiệm của bạn. Kiểm tra kết nối Wi-Fi hoặc dữ liệu di động trước mỗi trận đấu để đảm bảo bạn không bị gián đoạn. khi tận hưởng.

Bạn cũng cần lưu ý, tránh thoát ra khỏi trang web hoặc tắt trình duyệt liên tục trong quá trình xem trận đấu. Sử dụng nút tạm dừng hoặc tính năng tương tự để duy trì trải nghiệm mượt mà và không làm phiền người xem khác. Điều này giúp bạn tận hưởng trận đấu cực đã, linh hoạt nhất.
Như vậy, bài viết đã bật mí những thông tin hấp dẫn nhất về RakhoiTV. Qua đó, bạn sẽ có được những khoảnh khắc tận hưởng hấp dẫn cùng với đam mê và có được nhiều thông tin thể thao hữu ích.